Flash and fire points denote the temperatures at which a combustible substance gives sparks and fires respectively when ignited. They play a major role in the transportation of these fuels.
Safety is the most important term in every industry. An unsafe product or service affects not only the society but also it’s a shame to the engineer who designed it. For handling and categorizing fuels, Flash and fire point is very important. Before starting, you should not confuse these temperatures with auto-ignition temperature. I have discussed it already click here.
Table of Contents
Flash Point:
Flash point refers to the temperature at which when a burning matchstick is bought close to the fuel it produces spark and this spark will not be good enough to ignite the fuel. This temperature is different for different substances. And external heating can be provided to attain this temperature (for substances which has flash point higher than the room temperature).

Fire Point:
This happens when the temperature of the fuel goes even higher. Here, when you bring a matchstick close to the fuel, it will ignite. Fire will not stop until you cut the oxygen supply.
Why this happens?
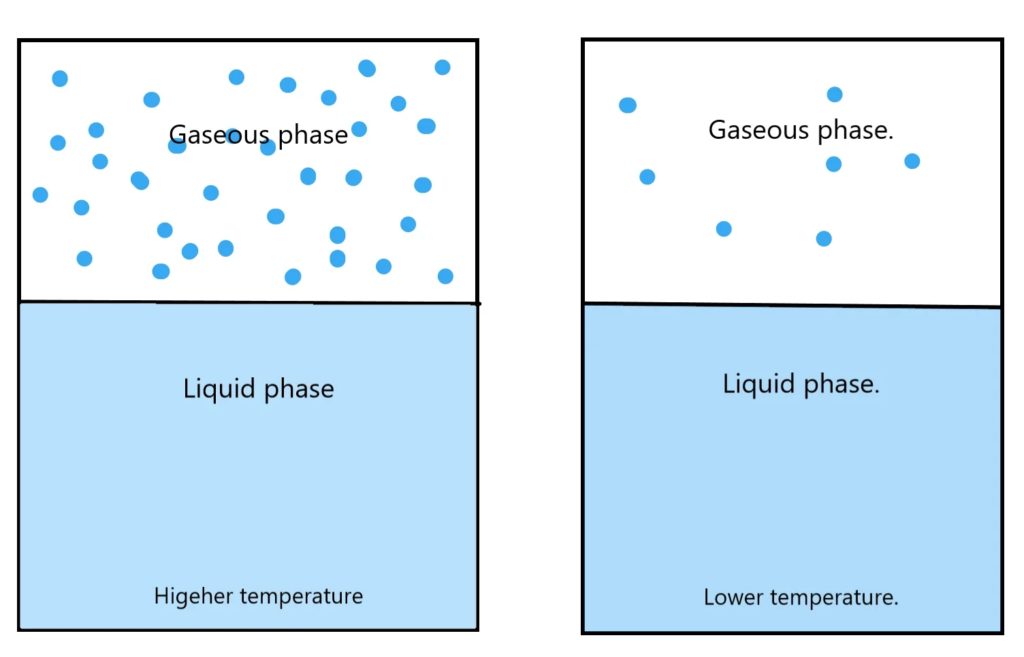
The reason for this phenomenon is vapour pressure. When a substance in liquid form is placed in a closed container, there is an equilibrium that exists between the substance in a liquid state and in a gaseous state. A few of the molecules on the surface of the liquid escape from and remain in its gaseous state. The pressure exerted by these vapours is known as vapour pressure. This mainly depends on two factors which are the temperature of the substance and the substance (Volatility).
The temperature limits the maximum vapour pressure exerted by a particular substance and it increases with temperature. Therefore, when the temperature reaches the flashpoint of the substance, the corresponding vapour pressure is just enough to combine with oxygen in the atmosphere and produce a spark. But it cannot last forever as the vapour pressure is not enough for the fire to propagate. Now, increasing the temperature. The vapour pressure also increases and when the fire point is reached, the vapour pressure is enough to help in propagating the flame and the fuel burns.

Applications:
These values are to design fuel tanks, fuel pipelines, etc. Since these temperatures are close to the atmospheric temperatures, we should take care while designing them. Also, it helps in classifying fuel as flammable and combustible. Flammable substances are those which has low flash points and burn vigorously. Whereas, combustible substances burn less vigorously and have relatively higher flash points. A flashpoint of 37.8 degrees celsius is taken to differentiate between the two.
