Battery electric vehicle (BEV) is the most viable form of transportation we have for the foreseeable future. But if we dig deeper into battery electric vehicles, we may find that there are a lot of variations within. From old school lead-acid batteries to modern metal-air batteries, BEVs have gone way too far. In this post, I will be digging deeper into the ‘battery’ part of BEVs.
Before I start, let me introduce a couple of basic terms which will be useful henceforth.
Specific energy is the amount of energy you can pack per kilogram. It is measured in Megajoules per kilogram (MJ/kg) or watt-hour per kilogram (Wh/kg). In simple words, more is the specific energy lighter the battery gets. When you measure the same with volume, we call it energy density. More is the energy density, the lesser the space the battery occupies. These two factors are pivotal to an electric vehicle.
The cathode is the negative electrode and the anode is the positive electrode.
Table of Contents
Batteries used in Electric vehicles
1. Lead Acid batteries
Lead-acid batteries are not the best option for EVs. Although, it is used in standard IC engine vehicles, household and commercial power backup systems. The problem with these batteries is that it has very low energy density and specific energy. Lead-acid batteries has about 35 to 40 WH/kg (0.126 to 0.144 MJ/kg) and 80 to 90 WH/L (0.288 to 0.324 MJ/kg). When comparing this with modern lithium-ion batteries, these batteries are terrible. This means, the range of your electric vehicle tanks.

But there are a handful of electric scooters in India that are powered by Lead-acid batteries. The main reason for it is the pricing of these batteries. They are very cheap compared to other modern batteries. But these vehicles are sluggish, and depending on the weight of the rider, some scooters cannot go more than 20 kmph!
As the name suggests, a Lead-acid battery consists of lead and acid. It has a porous and spongy lead cathode. It is porous and spongy to accommodate ion formation. On the other end, it has a lead oxide anode. Diluted sulphuric acid (sulphuric acid and water) acts as the electrolyte. Take a look at the chemical reactions.
At Cathode:
Pb(s) + HSO4−(aq) → PbSO4(s) + H+(aq) + 2e−
At Anode:
PbO2(s) + HSO4−(aq) + 3H+(aq) + 2e− → PbSO4(s) + 2H2O(l)
Overall Chemical reaction:
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
It is clear from the above reactions that lead sulfate is formed on both electrodes leaving water in the aqueous medium behind. This happens when the battery is completely out of juice. Therefore, it needs charging and the reverse process happens while charging.
2. Nikel-Metal Hydride NiMH batteries
Things got more interesting when auto engineers started playing with NiMH batteries. This is the battery that made up the hybrid market. Many hybrids notably the Toyota Prius was powered by this battery.

NiMH is almost 3 times more energy-dense than nickel-cadmium batteries. Most importantly, like the latter, it doesn’t contain cadmium. Hence it is less toxic. But it is not as good as Li-ion batteries making it less popular among modern EVs.
- Cathode: Nickel hydroxide
- Anode: Hydrogen absorbing alloys (Metal hydride)
- Electrolyte: Potassium hydroxide
- Specific energy: 60 to 120 Wh/kg (0.216 to 0.432 MJ/kg)
- Energy density: 140 to 300 Wh/l (0.504 to 1.08 MJ/l)
Cathode reaction:
H2O + M + e− ⇌ OH− + MH
Andode reaction:
Ni(OH)2 + OH− ⇌ NiO(OH) + H2O + e−
Overall reaction:
M + Ni(OH)2 ⇌ MH + NiO(OH) (Where MH is metal hydride)
3. Lithium-ion batteries
Lithium-ion batteries have revolutionized the world. Now the world fits in your pocket thanks to this technology. All gadgets ranging from laptops, smartphones, wearable tech and much more are powered by Li-ion batteries.

The reason why this became very popular is due to the battery’s specific energy and energy density. Currently, this is the best battery we can find in the market. And for the same reason, Li-ion powered electric vehicles are ruling the transportation industry.
Cathode: Lithium-based (Lithium cobalt oxide, Lithium Manganese oxide, Lithium-ion phosphate, Lithium-nickel based, etc)
Anode: Graphite or Lithium-based
Electrolyte: Organic (mostly)
Specific energy: 1 MJ/kg (Best case batteries)
Energy density: ~1.5 MJ/L (Best case batteries)
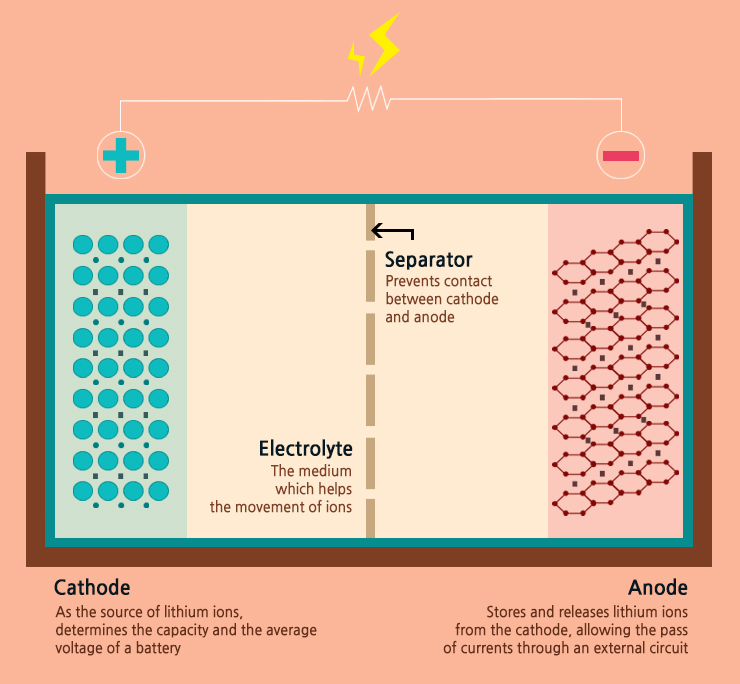
Advantages:
Lithium being the third lightest atom and lightest solid and metal, we have great energy packing efficiencies. Li’s small size also means it has lower internal resistance within the system. Hence the terminal voltage is 3.6 V which is one of the best. This means it can handle high-power applications as lesser current is drawn.
On top of that, it has no memory effect (A phenomenon where the battery’s charge cycle is depleted – More on it later). It requires less maintenance, a low self-discharge rate (1% to 2% a month) and is non-toxic.
Disadvantages:
On the darker side, it has overheating tendency leading to combustion and thermal runaway. Remember the Boeing 787 AKA the Dreamliner grounding issue? Even Samsung Galaxy Note 7 had issues. Therefore we need more safety equipment to protect them which would eventually add weight.
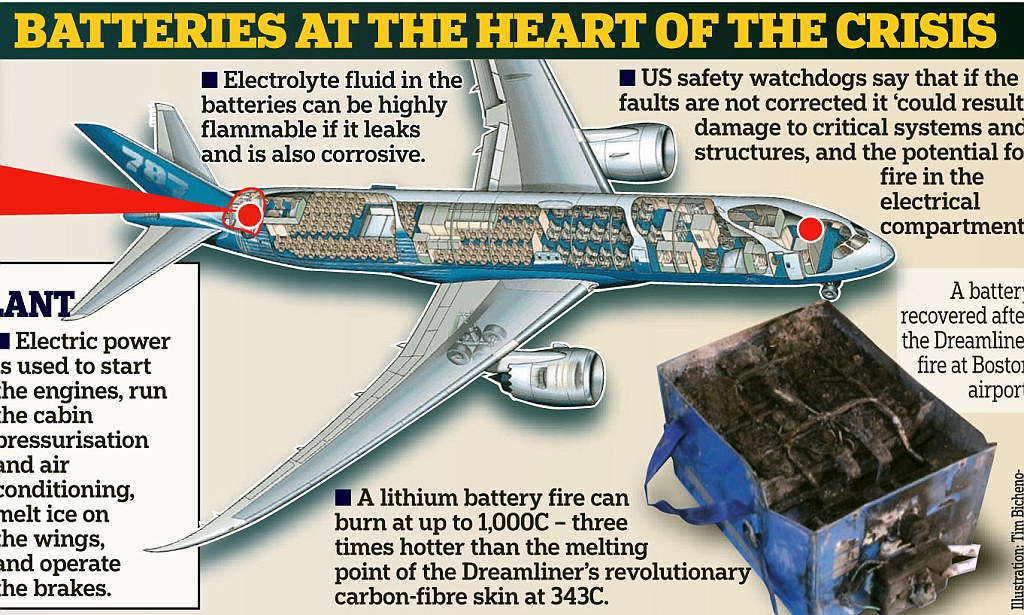
Although these batteries have lots of energy content, it is still 43 times lower than petrol or diesel. This is why lots of research is going to improve battery chemistry.
I am limiting the discussion on Li-ion here as I need a separate article just for Li-ion alone. Where I shall discuss the importance of cobalt, Nickel etc. I am working on it and stay tuned!
Batteries of the future
As I already stated, current batteries are about 43 times less energy-packed than traditional fuels and about 200 times lesser than hydrogen. This is the reason why we are looking for alternatives in the battery industry. I shall introduce you to solid-state and metal-air batteries.
1. Solid-state batteries
In a conventional battery, the electrolyte is in a liquid state. This makes them big and heavy. Therefore, experts are looking for alternatives that are light and consume lesser space. In a solid-state battery, the electrolyte is solid or semi-solid. This improves the specific energy and energy density drastically.
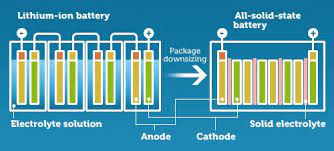
These batteries are less prone to fire hazards as there won’t be any swelling or electrolytic leakage (Ceramic based electrolyte used which is non-flammable generally). Also, these batteries have longer cycle life and this is due to a wider electrochemical window.
In simple terms, the electrochemical window defines the degree of undesirable reactions. If a battery has a good electrochemical window, the electrolyte is very stable. the electrolyte does not take part in undesirable redox reactions deteriorating the battery life.
Just like Li-ion batteries, I shall cover SSBs in a future blog.
2. Metal air batteries
Metal-air batteries have a very bright future. The cathode being air, is super light and can provide promising results. Currently, it is under development. The most common types are Lithium, Aluminium and Zinc-air. Let me give basic info about them here.
The cathode being air, oxygen enters the system and reacts with water (from electrolyte) molecules to form OH– ions these ions combine with the metal anode to form metal hydroxides leaving out electrons.
Cathode: Air (Oxygen)
Anode: Zinc, Aluminium, Lithium metal
Electrolyte: diluted potassium or lithium hydroxide
Specific energy: 5,000 to 11,000 WH/kg (18 to 39 MJ/kg) (Theoretical values)
Cathode reaction:
2H2O + O2 + 4e− ⇌ 4OH−
Anode reaction:
Al + 3OH– ⇌ Al(OH)3 + 3e–
Overall reaction:
3Al + 3O2 + 6H2O ⇌ 4Al(OH)3
Stay tuned for more info on metal-air batteries. Until then, you can check out other articles on electric vehicles – Click here.
